Seven years ago this month, medical cannabis became legal in the UK — a feat hard won by families and advocates who had experienced the life-changing potential of cannabis-based treatments.
Since then, the regulations have remained largely unchanged, but what’s grown around them is extraordinary: a sector built by clinicians, scientists, manufacturers and advocates determined to make treatment genuinely accessible for the patients who need it.
As the anniversary arrives, it’s a chance to look back on how far the UK’s medical cannabis ecosystem has come, and how collaboration has turned potential into progress.
The day the law changed
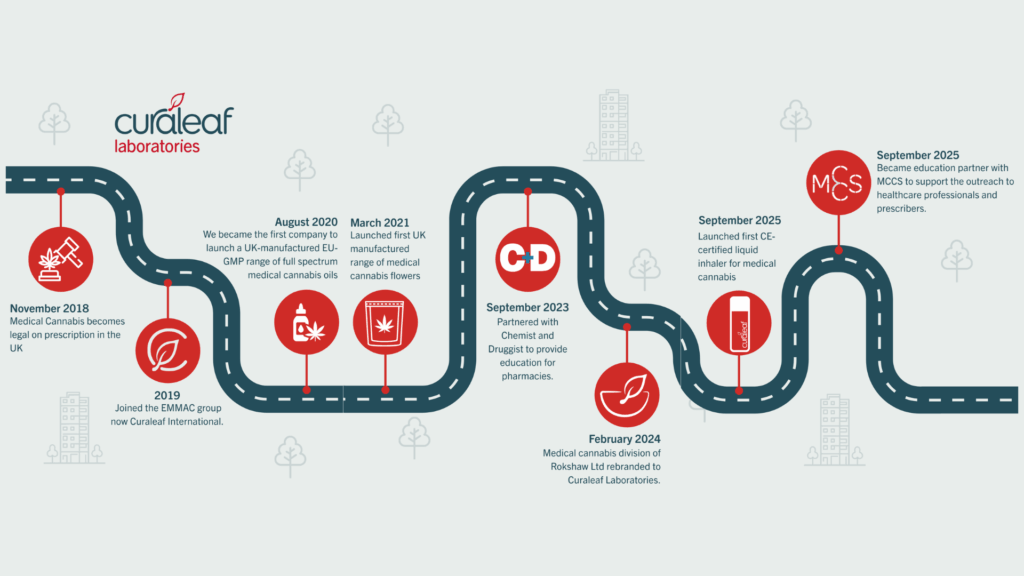
After almost a century of prohibition, November 1st, 2018, marked the day cannabis-based products for medicinal use were moved to Schedule 2 of the Misuse of Drugs Regulations, allowing specialist doctors to legally provide prescriptions in the UK.
The change followed years of campaigning by families, whose cases helped show that these medicines could be life-changing for children with severe epilepsy.
That public compassion reshaped policy. What had once been a taboo subject entered mainstream debate, and for a moment, the UK seemed poised to lead a new era in cannabinoids.
As the seventh anniversary arrives, it also carries a note of remembrance for Hannah Deacon, mother to Alfie, whose tireless advocacy helped make that moment possible. Her legacy continues to inspire campaigners, clinicians, producers and partners working together to expand access today.
What happened next?
The initial optimism in 2018 was tempered by the realities of regulation. Within months, patients and clinicians realised that access through the NHS would be far more limited than first imagined. Prescriptions were technically possible, but in practice almost unattainable.
Into the gap stepped a small group of specialist clinics and pharmacies, driven to make legal access a reality. The first private medical cannabis clinic opened in 2019, creating a model that would soon expand across the country. Over time, these clinics built the infrastructure the system needed: governance frameworks, clinical protocols, and data registries to track patient outcomes.
Domesticating the supply
As patient numbers grew, so did the need for reliable, high-quality supply. Initially, most products were imported from established EU-GMP manufacturers in Europe and Canada, ensuring pharmaceutical consistency, but with significant cost and shaky supply chains. It became clear that the UK would benefit from its own manufacturing base.
That’s when domestic production began to catch up. Experienced Specials manufacturers, including the team that would become Curaleaf Laboratories, brought cannabinoid medicines onshore, applying the same GMP principles and quality systems already used across the unlicensed medicines sector.
Today, those early efforts to streamline supply and improve access have been rewarded with genuine patient engagement, with around 60,000 private patients a year now receiving medical cannabis in the UK – and counting.
Building the foundation: Curaleaf Laboratories’ contribution
Curaleaf Laboratories, the medical cannabis division of Rokshaw Ltd, joined Curaleaf International in 2019. For the team, the shift into medical cannabis was a natural extension of over a decade spent producing high-quality, small-batch specials in the UK. With the necessary expertise and licences already in place, Curaleaf Laboratories were the first to manufacture medical cannabis domestically – helping shorten supply chains and stabilise costs.
Innovation through manufacture
By 2020, Curaleaf Laboratories launched the first UK-manufactured EU-GMP full-spectrum cannabis oils, earning national attention and setting a benchmark for consistent quality. The following year brought the country’s first UK-made medical cannabis flower range, further expanding treatment options.
Innovation continued at pace. Over the next few years, Curaleaf Laboratories introduced new dosage forms – including pastilles, oils, capsules, liquid vape cartridges , and the UK’s first CE-certified liquid inhaler for medical cannabis in 2025. Each development reflected the same goal: to make standardised, high-quality medicines accessible and clinically practical.
Behind these launches was a growing network of international partnerships, such as collaborations with Khiron Europe and ECS Botanics as well as domestic cultivation with Dalgety Ltd, all helping to strengthen a resilient, local supply chain that meets international GMP standards.
Educating the new generation of prescribers
Through partnerships with the Medical Cannabis Clinicians Society (MCCS) and Chemist + Druggist, Curaleaf Laboratories has supported education for healthcare professionals nationwide. More than 200 events have reached over 50,000 clinicians, pharmacists, and nurses to build confidence in an area that once lacked structured guidance.
Seven years on, Curaleaf Laboratories now supports 12 partner brands and distributes products across five dosage forms and growing — evidence not just of market maturity, but of a sector that’s increasingly united by shared standards and purpose.
Looking ahead
The regulations that reshaped UK medicines policy in 2018 have yet to evolve — but everything around them has. Infrastructure, education, and clinical engagement are now well-established, and the evidence base is expanding through randomised trials and large-scale registries.
For Curaleaf Laboratories and our partners, the next phase is about deepening that evidence and building trust across every part of the care pathway.
“What’s been achieved so far is something for us all to take pride in,” says Jonathan Hodgson, Managing Director UK at Curaleaf International. “It’s the result of persistence, collaboration and a shared belief that access to high-quality cannabis-based medicines has a place in modern healthcare.”